Shielding oxygen production to keep hydrogen coming
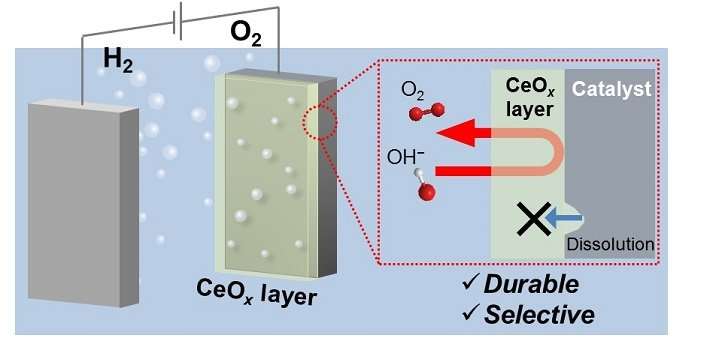
A porous cerium-based coating boosts the durability of oxygen-forming catalysts while maintaining their inherent water-splitting activity.
The efficiency of separating hydrogen and oxygen from water could be boosted using cost-effective electrocatalysts that combine high performance and robustness. Researchers from KAUST have developed a coating that protects water-splitting catalysts dedicated to so-called oxygen evolution without reducing their electrochemical activity.
Hydrogen carries substantial energy. Extensive efforts to isolate from water this promising source of clean fuel have led to electrochemical approaches, such as water electrolysis and light-driven water splitting. These methods typically rely on hydrogen formation at the cathode and oxygen evolution at the anode. However, unlike its hydrogen counterpart, oxygen evolution is usually slow and requires significant overpotential, which means that the anode consumes more energy than thermodynamically estimated. This hinders the overall hydrogen production.
Nickel–iron-oxide catalysts, such as NiFeOx, have proven highly active for the anodic reaction but are unstable under harsh oxidative conditions. Exposure to excess voltage or alkaline solutions causes these catalysts to lose iron-containing species, which dissolve from the active sites and gradually become deactivated.
To address this issue, Ph.D. student Keisuke Obata and Professor of Chemical Science Kazuhiro Takanabe have developed a protective coating for a NiFeOx anode. The thin cerium-oxide (CeOx) layer consisted of tiny particles aggregated into a porous structure that allowed only generated oxygen molecules to escape.
According to Takanabe, his research group generally introduces nanoscale functional features on the surfaces of water-splitting electrodes to enhance performance. "Our group has found many nanostructured coatings for the hydrogen evolution cathode, but none that could stably cover the surface of the oxygen evolution anode," he says.
Building on their experience on the cathodic coatings, the researchers came up with a new coating for the oxygen evolution catalyst: anodic deposition of Ce3+ ions, which were oxidized and precipitated as a CeOx layer under applied voltage. "We did not expect the cerium species to improve the stability of the nickel–iron-oxide catalysts at the beginning of the project," says Obata, noting that while stability is an important factor for catalysis reactivity is critical.
The CeOx layer maintained the intrinsic reactivity of the electrocatalyst underneath and, consequently, only added selective permeability and durability to the electrode. A similar phenomenon occurred for a CeOx-coated anodic cobalt electrocatalyst, which means that this deposition will work across various oxygen evolution catalysts.
Takanabe's team is currently investigating different materials to perfect the properties of their coating. "We are testing our materials under industry-relevant conditions," he adds.
More information: Keisuke Obata et al. A Permselective CeO x Coating To Improve the Stability of Oxygen Evolution Electrocatalysts, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201712121
Journal information: Angewandte Chemie International Edition


















