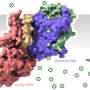
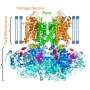
Stop and go in the potassium channel

Cells need openings in the cell membrane in order to make exchanges with their environment. These openings are closable portals in which the signals are transported in the form of ions. Private lecturer Dr. Indra Schröder from the Department of Membrane Biophysics at the TU Darmstadt, which is run by Professor Gerhard Thiel, is interested in potassium channels. The physicist and head of the junior research group has her very own view of these tiny molecular machines. She is not so much interested in the biological signals that are exchanged via the channels, but in the biophysical closing mechanism. Schröder wants to know what the molecular bolt looks like and how it works.
To that end, the physicist works with very basic potassium channels so as not to make the analysis unnecessarily complicated. She uses two systems with a similar structure but different opening probabilities. One channel is almost always closed, the other almost always open. Both channels originated from algae viruses, but strongly resemble the potassium channels of higher organisms. Schröder works in a cell-free system, and fits the potassium channels into artificial surfaces. "We concentrate solely and exclusively on the closing mechanism, and blend out all the other functions of the potassium channels," says Schröder. "This is legitimate, because the potassium channels are all similar to each other. We are basically working on a prototype, what you could call a model potassium channel."
Amino acid serine at a critical point
Schröder and her doctoral student Oliver Rauh have mutated both potassium channels and pushed individual parts of them to and fro like pawns. This enabled them to establish what amino acids are responsible for the low opening probability, and which for the high. The potassium channel with the low opening probability possesses the amino acid serine at a critical point. This amino acid interacts with a remote amino acid, which forces the channel pore to bend. This curve folds another amino acid into the transport route that closes the tunnel.
With the potassium channel with the high opening probability, the amino acid serine was replaced by the amino acid glycine. Glycine does not force the channel pore to bend, which means the tunnel does not close either. "So the closing mechanism of these two viral potassium channels consists only of two amino acids," explains Schröder."One amino acid closes the channel, the other controls the process. Molecular-dynamic simulations by our co-operation partner Stefan Kast of the TU Dortmund have confirmed this. We had been expecting a far more complicated closing mechanism."
The head of the junior research group assumes that some human potassium channels possess the same bolt as the two viral potassium channels because they both possess the two critical amino acids. Schröder's work is also of relevance to synthetic biology because the potassium channels and their basic closing mechanism can be used in the construction of artificial nano sensors. The noMagic project, in which Schröder is involved, was financed by the European Research Council (ERC), and manipulates living cells with artificially manufactured channels.
More information: Oliver Rauh et al. Identification of Intrahelical Bifurcated H-Bonds as a New Type of Gate in K+ Channels, Journal of the American Chemical Society (2017). DOI: 10.1021/jacs.7b01158
Journal information: Journal of the American Chemical Society
Provided by Technische Universitat Darmstadt