Safe lithium-metal batteries with graphene
Recently, researchers at Tsinghua University, China have proposed a graphene-based nanostructured lithium metal anode for lithium metal batteries to inhibit dendrite growth and improve electrochemistry performance. They report their findings in Advanced Materials, published on March 16, 2016.
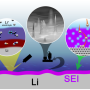
"Widely used lithium-ion batteries cannot satisfy the increasing requirement of energy storage systems in portable electronics and electric vehicles. New lithium metal anode batteries, like Li-S and Li-air batteries, are highly sought. Lithium metal provides an extremely high theoretical specific capacity, which is almost 10 times more energy than graphite," said Prof. Qiang Zhang, at the Department of Chemical Engineering, Tsinghua University. "However, the practical applications of lithium metals are strongly hindered by lithium dendrite growth in continuous cycles. This induces safety concerns. The lithium dendrites may cause internal short circuits resulting in fire. Furthermore, the formation of lithium dendrites induces very low cycling efficiency." The dendrite growth and unstable solid electrolyte interphase consume large amount of lithium and electrolyte, and therefore leading to irreversible battery capacity losses. Consequently, inhibiting the dendrites growth is highly expected.
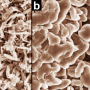
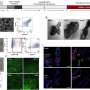
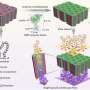
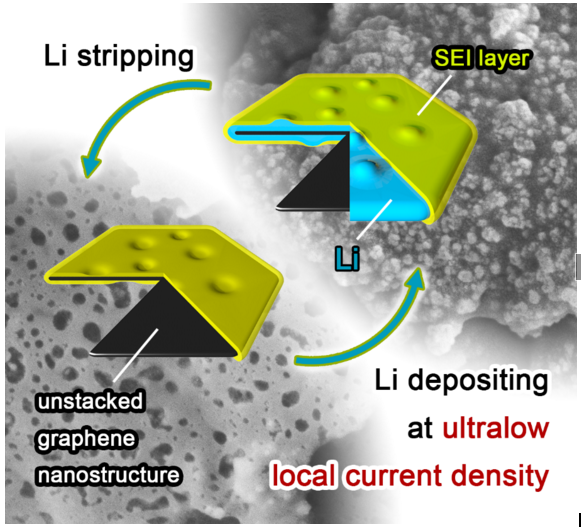
Many approaches have been proposed to retard the growth of dendrites through electrolyte modification, artificial solid electrolyte interphase layers, electrode construction, and others. "We noticed that by decreasing the local current density heavily, lithium dendrite growth could be efficiently inhibited. Based on this concept, we employed unstacked graphene with an ultrahigh specific surface area to build a nanostructured anode. And it turned out to be a very efficient idea," said Rui Zhang, a Ph.D. student and the first author. "Additionally, we have employed the dual-salt electrolyte to acquire more stable and more flexible solid electrolyte interphase, which can protect the lithium metal from further reactions with electrolyte."
This graphene-based anode offered great improvement, including (1) ultralow local current density on the surface of graphene anode (a ten-thousandth of that on routine Cu foil-based anodes) induced by the large specific surface area of 1666 m2 g-1, which inhibited dendrite growth and brought uniform lithium deposition morphology; (2) high stable cycling capacity of 4.0 mAh mg-1 induced by the high pore volume (1.65 cm3 g-1) of unstacked graphene, over 10 times of the graphite anode in lithium-ion batteries (0.372 mAh mg-1); (3) high electrical conductivity (435 S cm-1), leading to low interface impedance, stable charging/discharging performance, and high cycling efficiencies.
"We hope that our research can point out a new strategy to deal with the dendrite challenge in lithium metal anodes. The ultralow local current density induced by conductive nanostructured anodes with high specific surface area can help improve the stability and electrochemistry performance of lithium metal anodes," said Xin-Bing Cheng, a co-author of the work. Future investigation is required to design preferable anode structures and to produce more protective solid electrolyte interphase layers. The researchers also call for additional study of the diffusion behavior of Li ions and electrons in the process of lithium depositing and stripping to advance the commercial applications of lithium metal anodes.
More information: R. Zhang, X.-B. Cheng, C.-Z. Zhao, H.-J. Peng, J.-L. Shi, J.-Q. Huang, J. Wang, F. Wei, Q. Zhang. Conductive Nanostructured Scaffolds Render Low Local Current Density to Inhibit Lithium Dendrite Growth. Adv. Mater. 2016, 28, 2155-2162. DOI: 10.1002/adma.201504117.
Journal information: Advanced Materials
Provided by Tsinghua University